Unveiling the Enigmatic Elixirs: A Creative Odyssey into the World of Solvents
Embark on an enchanting odyssey into the world of solvents, where alchemy meets everyday life. Explore their mystical origins, the harmonious symphony of molecular dance, and their roles in industries, art, and cleanliness. From ethereal elixirs to environmental waltzes, discover the transformative magic of solvents
Unveiling the Enigmatic Elixirs: A Creative Odyssey into the World of Solvents
The Alchemy of Solvents
In the vast laboratory of chemical wonders, solvents stand as enigmatic elixirs, performing a dance of dissolution and transformation. Join us on a creative odyssey as we explore the kaleidoscope of solvents, from their ethereal origins to their diverse roles in dissolving the barriers between substances.
The Alchemical Prelude
The stage is set with the alchemical prelude, introducing solvents as mystical concoctions with the power to dissolve, disperse, and unveil hidden secrets. Act I takes us back to the ancient laboratories where alchemists first discovered the enchanting properties of these liquid alchemies, setting the foundation for the transformative journey ahead.
The Solvent Symphony
As the alchemical prelude fades, Act II unfolds the solvent symphony. Each solvent, like a musical note, possesses unique properties and interactions. From water's universal solvency to the organic cadence of ethanol, the symphony explores the harmonious dance of molecules as they dissolve and create new compositions. Solvents become the conductors orchestrating the grand ballet of dissolution.
Ethereal Elixirs and Everyday Alchemy
Act III delves into the ethereal elixirs that have transcended the alchemical realms into everyday life. From acetone's magic in nail polish removal to the soothing touch of rubbing alcohol, everyday alchemy becomes a reality. The solvents that grace our households reveal the practical enchantment woven into the fabric of our daily experiences.
Solvent Symbiosis in Industry
Enter Act IV, where solvents take center stage in industrial realms. In this act, the spotlight shines on the crucial roles solvents play in manufacturing, from pharmaceuticals to paints. The solvent symbiosis unfolds, showcasing their ability to dissolve barriers and forge new alliances between raw materials, giving birth to products that shape our modern world.
The Dance of Cleanliness and Dissolution
Act V explores the dance of cleanliness and dissolution, revealing the role of solvents in cleansing agents. From the gentle touch of soap to the potent embrace of degreasers, solvents become the choreographers of cleanliness, dissolving impurities and unveiling the pristine surfaces beneath. It's a dance that transcends the physical, embodying the purity of molecular movement.
The Environmental Waltz
As the odyssey continues, Act VI leads us into the environmental waltz of solvents. Here, the narrative unfolds both the benefits and challenges of solvents in our ecosystems. From the sublime role of water in sustaining life to the discordant notes of environmentally harmful solvents, the waltz explores the delicate balance between solvent utility and ecological responsibility.
Solvents in Artistic Alchemy
In Act VII, the spotlight turns to solvents as tools of artistic alchemy. From the brush strokes of oil painting to the vivid hues of ink, solvents become the alchemists' accomplices, manipulating pigments and textures on the canvas. The creative spirit merges with molecular magic, leaving behind masterpieces that captivate the senses.
The Evanescent Finale
As the odyssey approaches its evanescent finale, Act VIII unravels the transient nature of solvents. Their ability to dissolve and transform, once a mysterious dance, now takes center stage in the finale. The ephemeral quality of solvents becomes a metaphor for their ever-changing role in the grand theater of molecular interactions.
Frequently Askied Questions
What do you mean by solvent? A solvent is a substance, typically liquid, capable of dissolving another substance (solute) to form a homogeneous solution. Solvents are often used in processes like dissolving, cleaning, or extracting other substances.
What does in solvent mean? It seems like there might be a typo in your question. If you meant "What does 'in solvent' mean?" it would depend on the context. If a substance is "in solvent," it suggests that the substance is dissolved or present within the solvent.
What is an example of a solvent? Water is a classic example of a solvent. Other common solvents include ethanol, acetone, and various organic compounds.
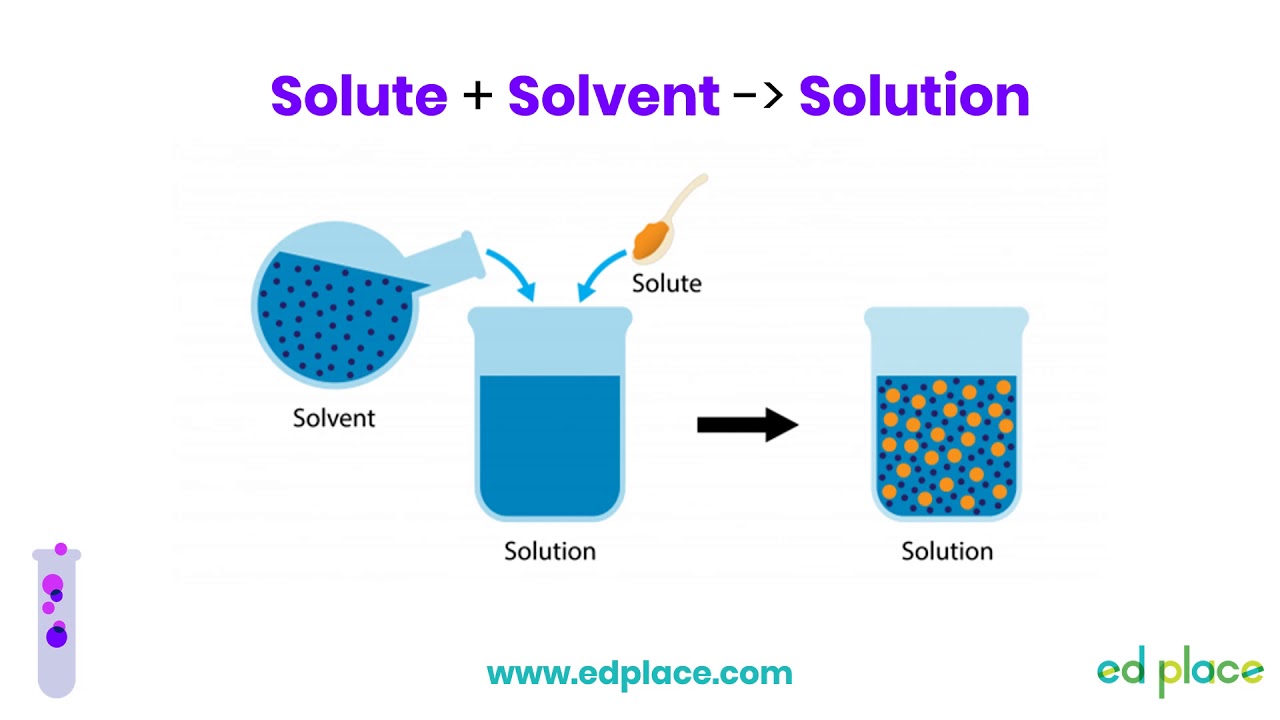
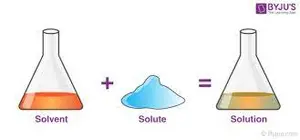
What is the difference between solute and solvent? A solute is a substance that is dissolved in a solvent to form a solution. The solvent is the substance that does the dissolving. In simple terms, solute is what gets dissolved, and solvent is what does the dissolving.
What is the difference between a solute and a solvent in tabular form?
| Parameter | Solute | Solvent |
|---|---|---|
| Definition | Substance being dissolved | Substance doing the dissolving |
| Example | Salt in saltwater | Water in saltwater |
| Role | Gets dissolved | Does the dissolving |
What is the definition of solute and solvent easy? In simple terms, a solute is what gets dissolved, and a solvent is what does the dissolving. For example, in saltwater, salt is the solute and water is the solvent.
What are the 5 examples of solute?
- Salt
- Sugar
- Oxygen (in air)
- Carbon dioxide (in soda)
- Ethanol (in alcoholic beverages)
Is Salt a solute or solvent? Salt is a solute. In a saltwater solution, salt is dissolved in water, making water the solvent.
Is water a solute or solvent? Water is typically a solvent. It dissolves many substances (solute) to form solutions.
Is milk a solute or solvent? Milk is a complex mixture of water, proteins, fats, and sugars. In the context of water and milk, water can be considered a solvent, and the other components are solutes.
Is sugar a solute or solvent? Sugar is a solute. When sugar dissolves in water, water is the solvent.
Is alcohol a solute or solvent? Alcohol can play both roles. It can act as a solute when mixed with water and as a solvent when dissolving other substances.
Is kerosene a solute or solvent? Kerosene is typically considered a solvent. It can dissolve certain substances but is not as versatile as water in this regard.
Is beer a solute? Beer is a complex mixture of water, alcohol, hops, malt, and other compounds. In the context of water and beer, water can be considered a solvent, and the other components are solutes.
Is Wine A solute? Similar to beer, wine is a complex mixture. Water in wine can be considered a solvent, and the various components, such as alcohol and flavor compounds, are solutes.
Is HCl a solute? Hydrochloric acid (HCl) can act as a solute when dissolved in water.
Is steel a solvent or solute? Steel is neither a solvent nor a solute in the traditional sense. It is a solid alloy composed mainly of iron and carbon.
Is water a solute in milk? Water in milk can be considered a solvent. Milk is a colloidal suspension of fat globules and proteins in water.
Is milk 100% water? Milk is not 100% water. It contains water along with proteins, fats, lactose, minerals, and vitamins.
Does Dettol dissolve in water? Dettol, being an antiseptic solution, can dissolve in water. It forms a homogeneous solution when mixed with water.
Is oil a solute or solvent? Oil is typically considered a solute in the context of mixing with water, where water acts as the solvent.
Is glucose is a solute? Yes, glucose is a solute. It dissolves in water to form a solution.
Is petroleum a solvent? Petroleum itself is not a solvent. It is a complex mixture of hydrocarbons. However, certain refined petroleum products can act as solvents.
Is paint a solvent or solute? Paint is a complex mixture of pigments, binders, solvents, and additives. In the context of the solvent, the liquid component that evaporates, it is considered a solute.
What are 2 types of solvents?
- Water
- Organic solvents (e.g., ethanol, acetone)
What are 3 types of solvents?
- Water
- Organic solvents (e.g., methanol, hexane)
- Inorganic solvents (e.g., sulfuric acid)
Does solvent mean rich? No, in the context of chemistry, "solvent" refers to a substance capable of dissolving other substances. In financial terms, "solvent" describes an individual or entity that has sufficient assets to cover its liabilities and debts. The two meanings are unrelated.
The Alchemy Continues
The final curtain falls, but the alchemy of solvents continues. From the ancient laboratories to modern industrial landscapes, from the canvas of artistic expression to the everyday products that grace our lives, solvents persist as transformative agents. The odyssey may end, but the enchantment of solvents, the alchemical dance of dissolution and creation, remains an ever-present symphony in the grand tapestry of chemistry.